The Clever Coatings of Coronavirus
It’s been months since the infamous coronavirus has crept across the globe, closing schools and workplaces and changing the way we live our lives. But why is COVID-19 seemingly so good at infecting people? What makes this virus different than others? We talk to undercover superhero, Rommie Amaro of the University of California San Diego, about her discoveries through computational simulation of what the virus actually looks like, how it moves, and what that means for each of us.
Credits
Interview with Dr. Rommie Amaro, University of California San Diego
Producers: Taylore Ratsep, Jolie Hales
Hosts: Jolie Hales, Ernest de Leon
Writer / Editor: Jolie Hales
Follow Dr. Rommie Amaro
- Twitter: Rommie Amaro
- Amaro Lab
- Link to Study – Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein
___
11-19-20 UPDATE:
Congratulations to Rommie Amaro and her team for winning a Gordon Bell Award (known as the Nobel Prize of supercomputing), for their COVID-19 research! Read more.
___
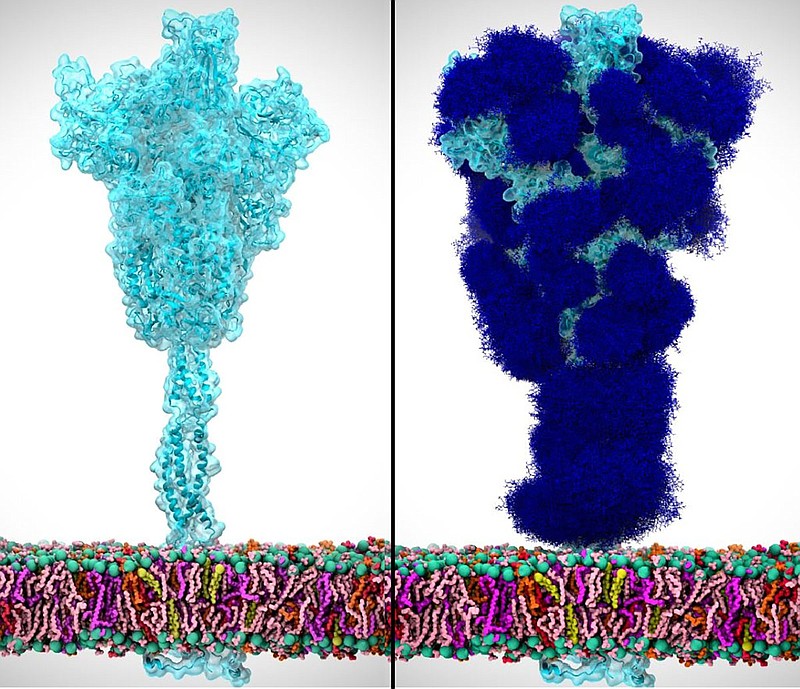
A molecular simulation of the Sars-CoV-2 spike protein shows how two key glycans (dark blue and green) orient themselves so they can push the receptor binding domain (turquoise) upward to infect cells.
More on Rommie’s Research
- Beyond Shieling: The Roles of Glycans in the SARS-CoV-2 Spike Protein
- Glycans on SARS-CoV-2 may help the virus infect cells – Alla Katsnelson, Chemical and Engineering News
- Coronavirus Is Covered In Sugar Shield, UCSD Scientists Figure Out What It Looks Like -Shalina Chatlani, kpbs (June 25, 2020)
- Frontera -A New NSF-Funded Petascale Computing System – Texas Advanced Computing Center
Jolie Hales:
If I saw marshmallow peep sugar doing that, it would freak me out.
Rommie Amaro:
You would eat it.
Jolie Hales:
Hello, everyone. I’m Jolie Hales.
Ernest de Leon:
And I’m Ernest de Leon.
Jolie Hales:
And welcome to the Big Compute podcast. [theme music]
Jolie Hales:
Here we celebrate innovation in a world of virtually unlimited compute, and we do it one important story at a time. We’re talking about the stories behind scientists and engineers who are embracing the power of high-performance computing to better the lives of all of us.
Ernest de Leon:
From the products we use every day to the technology of tomorrow, high performance computing plays a direct role in making it all happen, whether people know it or not.
Jolie Hales:
Yes. So on our last episode, we spoke about computational simulations that were run to see how COVID-19 spreads through the air indoors. And we talked about which musical instruments are at higher risk of spreading the disease.
Ernest de Leon:
Which included giving props to one of my favorite instruments, the sousaphone.
Jolie Hales:
We didn’t talk about the sousaphone.
Ernest de Leon:
It’s just a wearable tuba. That’s all it is.
Jolie Hales:
Oh! [laughs] Clearly I’m not a tuba player. Oh, okay. This sousaphone is a wearable tuba. Wow. I learned something and I minored in music. So that was a fail in my education.
Ernest de Leon:
Yeah. Any marching band you’ve ever seen, you know, the tubas that they kind of come up and face forward?
Jolie Hales:
Yeah. The ones that are all wrapped around them, like a snake?
Ernest de Leon:
That’s the sousaphone. Yeah.
Jolie Hales:
Ohhh… But it’s literally the exact same as the tuba other than the way you wear it?
Ernest de Leon:
Yep. Same key. Same everything.
Jolie Hales:
Wuuuut…
Ernest de Leon:
Named after John Philip Sousa. One of the most famous composers of all time.
Jolie Hales:
That makes sense. Huh. Thanks for teaching me something. [Sousa music clip] Today, we’re going to continue down a similar path, not so much with musical instruments, but with…
Newscasters:
The coronavirus — coronavirus — The coronavirus pandemic, because new cases are on the rise — The new numbers not seen since the summer–
Jolie Hales:
Which is unfortunately still very much on the forefront of our lives. It’s affecting our health, our schools, our places of worship, our workplaces. It’s really hard to find something that hasn’t been affected. I mean, as just an example, I worked for the Walt Disney Company for about eight years.
Disney Announcer:
Please welcome our Disney Ambassador, Jolie Hales!
Jolie Hales:
I represent the 23,000 cast members that work at the Disneyland Resort.
Interviewer:
That’s all?
Jolie Hales:
Well, maybe 23,001, I dunno.
Interviewer:
That extra one adds a lot.
Jolie Hales:
And I built a lot of really great relationships there because there’s a lot of amazing people who work for Disney. And then recently over a period of just a few hours, I literally watched on my Facebook feed as hundreds of my Disney friends lost their jobs.
Newscasters:
First with breaking news out of Disney. Disney is laying off 28,000 domestic employees at its parks experiences and product segment. And that will affect employees across all levels, hourly, salaried and executive roles.
Jolie Hales:
For some, it was the only job they ever knew. And it was this weird feeling to have this sadness kind of hanging over the “happiest place on earth.” And I know that other industries like airlines and theaters are seeing similar losses.
Ernest de Leon:
That’s true. As a matter of fact today, I was reading an article that Cisco is doing some massive layoffs right now. And the sentiment is that it’s probably the largest layoff they’ve ever had. Oh man. The thing about this virus or a virus in general is that it doesn’t care what your job is. It doesn’t care what your welfare social status is. It’s perfectly fine to invade your life regardless. Just look at the President of the United States.
Jolie Hales:
Yeah. People were kind of flipping out over that one.
President Donald Trump [clip]:
Wasn’t feeling so well. I feel much better now. We’re working hard to get me all the way back.
Jolie Hales:
So today we’re going to talk about an undercover superhero [superhero music] who is working with high-performance computing to do something that we are all rooting for: find a cure for this stupid virus, or at least understand it enough to get to a vaccine and better therapeutics so that it’s not so deadly and not so interruptive.
Ernest de Leon:
I think at this point, we’re all ready to just heal and get on with our lives.
Jolie Hales:
So true. So if you search the interwebs for COVID-19 research, the name Rommie Amaro pops up everywhere. And for good reason. Rommie is–
Rommie Amaro:
I’m a professor of chemistry and biochemistry at the University of California, San Diego.
Jolie Hales:
Where she works in–
Rommie Amaro:
what we call computational chemistry or computational biophysics. It’s basically that we are using mathematics and computing to understand better sort of biological and chemical systems.
Jolie Hales:
And not only is Rommie a kick-butt scientist, but she’s just an all-around cool human.
Rommie Amaro:
I have four children.
Jolie Hales:
Awesome.
Rommie Amaro:
So I spend a lot of time right now doing sort of the remote schooling business that we’re all, you know, many of us are sort of dealing with. I mean, one of the good things about having so many kids, is like, we’re not necessarily really lonely here. We have a lot going on. So that’s a good thing.
Jolie Hales:
It’s always a party at the Amaro home.
Rommie Amaro:
Exactly, exactly. Birthdays come and we can still have a crowd for singing. So that’s good.
Jolie Hales:
And when she can break away–
Rommie Amaro:
But I’m also a runner. Um, I’ve been a runner since I was really young and I enjoy the heck out of that. So I try to do that, you know, as often as I can.
Ernest de Leon:
Uh oh… There was an audible gasp there. I heard how excited you got to talk about running.
Jolie Hales:
Yeah. That got me a little off track for a bit. What’s your favorite distance to run?
Rommie Amaro:
You know, I think the best distance is a half marathon.
Jolie Hales:
That’s my favorite, too!
Rommie Amaro:
Oh my gosh!
Jolie Hales:
But after some solid blissful moments discussing the joys of repetitively putting one foot in front of the other until you almost collapse, Rommie told me that during the times that she’s not running or with the family, she splits her work time between teaching and doing research.
Rommie Amaro:
I have to say, we do spend quite a bit of time doing the research part of things.
Ernest de Leon:
I’d say that’s understandable given the state of things right now. Everyone wants to know how to put this pandemic to bed.
Rommie Amaro:
My group jumped into studying COVID-19 really with all feet, and it’s been so intense.
Jolie Hales:
And when she started this research, she began to see something in the scientific community that she had never really seen before.
Rommie Amaro:
By mid-March, we had already mobilized and said, “Hey folks, we all need to work together on this like we’ve never worked together before,” because typically, you know, academic researchers are kind of weird. Well, you know that, you’re like, yeah, tell me something I don’t know. But we tend to study things and be very possessive about them. We don’t share with people necessarily unless we really trust them or unless they’re collaborators. And we certainly wouldn’t share something very early. Like you get a little bit nervous about sharing things because hey, you might have a mistake in there. That’s part of it. People are insecure, but also there’s a competitive edge to it, right, where, like, you want to be first because in science it’s a lot about being first and making that first discovery. So there isn’t really a sense of sharing generally to the extent that probably we should, but that was completely not the case with COVID-19. And so we just said, as a community, actually we had over 200 different groups come together and say we’re going to share things as quickly as we can. We’re going to build these models together.
Jolie Hales:
Wow.
Rommie Amaro:
Yeah, so it’s been really cool. So we have had to stay really organized because we, we actually have shared this data now with people worldwide.
Ernest de Leon:
Yeah. That’s absolutely true. I was even surprised to see how this played out in terms of research and science. The scientist community decided that it wasn’t important to find out who started this or why. What was important was to figure out what was causing this and how could we solve it. So in this case, it was amazing to watch these scientists just come together globally, ignore all of the white noise, and just get to work on trying to find a solution for humanity.
Jolie Hales:
Yes. This kind of global collaboration in the scientific community, as you said, is kind of unprecedented. I mean, if you think about it, I don’t know. Maybe what makes the COVID situation different is that every scientist and researcher out there has family members and friends who are in that high-risk category for the virus. It pretty much hits close to home for everyone. And researchers know that it’s kind of on them to basically fight for the human race.
Ernest de Leon:
How did Rommie and her colleagues first get involved in this research? Were they working on something else and they dropped it to study COVID or were they looking for something else to study in particular?
Jolie Hales:
Well, the timing is pretty interesting Rommie and her team had actually just spent five or six years studying influenza — you know, that common flu that we’ve all had and that we all completely hate.
Ernest de Leon:
When you say “we’ve all had,” you must mean the royal we. I’ve never had the flu or a flu shot, but–
Jolie Hales:
What?! You’ve had the flu!
Ernest de Leon:
Never in my life have I had the flu or had to have a flu shot.
Jolie Hales:
What? You’re an anomaly, Ernest. Nobody who’s listening to this can relate to you. I’m telling you, I get the flu every year and I get a flu shot, so I don’t know what’s going on with me and my immune system. We need to study you, is what we need to do.
Ernest de Leon:
Yeah. I’ll donate my body to science once I’ve exited this world.
Jolie Hales:
Which hopefully won’t be anytime soon.
Ernest de Leon:
That’s the hope.
Newscasters:
How do you distinguish between coronavirus and the typical flu? It can be a challenge when a patient first presents for care because the symptoms overlap quite a bit.
Rommie Amaro:
There was some news about it already kind of trickling around, and my colleagues and I started just kind of watching what was happening in terms of spread.
Jolie Hales:
So they spent all these years putting together a study about influenza that was actually published in February, right when COVID was hitting the news in like a massive way.
Rommie Amaro:
Because at this point in early February, it had really started to take off in Italy.
Newscasters:
The prime minister has put a total lockdown for all 60 million people until next month. Italy has the biggest cluster of cases outside of China.
Jolie Hales:
And that was when we said, okay, I think we really need to sort of pivot our efforts and try to see what we can do with this. And then after that, it came very quickly to the United States and things really took off.
Ernest de Leon:
It sounds like they didn’t have a lot of time to celebrate the completion of their influenza study. They had to pivot nearly immediately from one study to another.
Jolie Hales:
Yes, so if they were expecting a break, they didn’t get one.
Ernest de Leon:
So what about COVID did they study?
Jolie Hales:
Ok — I’ll tell you, but before I do, I want to quickly review how viruses work.
Ernest de Leon:
Enlighten us.
Jolie Hales:
So, in a nutshell, viruses are infectious microorganisms that need a living host to survive or to multiply. So they’re obviously too small to see with the naked eye. In fact, according to Dr. Clayton Kal of the Mayo clinic, the COVID-19 virus is one-900th, the width of a piece of hair.
Ernest de Leon:
One-900th?
Jolie Hales:
Yes. So as we’re just going about our business out there in the world, if this tiny virus is able to get into our body, either through an infected person sneezing, or by licking the infected surface of a telephone pole for some reason–
Ernest de Leon:
Or, as we’re finding out with COVID, just standing indoors near an infected person who is talking, or even just breathing.
Jolie Hales:
Or an asymptomatic person playing the trumpet in the same room.
Ernest de Leon:
But not the tuba.
Jolie Hales:
Nope. Tuba’s good. So in any of these infectious situations, a virus can enter our body through the mouth or nose or whatever. And then it tries to attach itself to one of our living cells because that’s how it basically survives. It needs that living host. And if it’s successful, it does everything in its power to start multiplying and attach to more and more cells often making us feel like garbage along the way.
Ernest de Leon:
I’ve never had the flu myself. I have had bronchitis several times and I can tell you being sick with an upper respiratory infection is the worst.
Jolie Hales:
Oh, amen to that. And I’m still absolutely blown away that you’ve never had the flu. Like, seriously, scientists listening to this need to like get blood samples or something and figure out what the deal is because people like me want a piece of that action. I don’t know– I’ll take a blood transfusion from you, Ernest, if that’s going to cure me of all future flus. I don’t think that’s how science works.
Ernest de Leon:
I mean, it might prevent you from getting a viral infection, but bacteria? They’re safe.
Jolie Hales:
Gotcha. But I mean, it really is crazy how something, one-nine-hundredth the width of a piece of hair can have such a massive effect on us. But after the virus has infected us, if things go well, our body recognizes that we’ve been invaded, and then it launches a super epic immune response where this huge army of defender cells comes out of the woodwork, or I guess the bloodwork in this case, and sets off to seek and destroy all invaders.
Ernest de Leon:
So it’s like that scene from Lord of the Rings where you’re having the battle of Helm’s Deep and it looks like all is lost. And then all of a sudden Gandalf appears at the top of the ridge with the waherin behind him and the sun glaring down behind him onto the army of orcs.
Jolie Hales:
Yeah. It could be that. I like to picture more of like a space invader fight. I picture a Star Wars fight, but I like the Lord of the Rings take. Um, in fact, okay. So part of that immune response in our bodies is executed by what are known as T-cells. And you’ve probably heard of them before. They are these big players in this fight. Um, with my analogy instead of X-wings, we could call them T-wings.
Ernest de Leon:
Although there are X-wings and A-wings, T-wings really doesn’t have the same ring to it.
Jolie Hales:
Okay. You’re right. I guess it sounds more like a cartoon bird drinking tea or something. But anyway, you get the idea, right? These T-cells go to work, killing off the virus invaders. And then another part of our immune system, called the B cells, starts making antibodies, which are special proteins that basically have two main jobs. So first they bind themselves to the virus to stop them from multiplying. And second, they kind of stamp a label on the virus as being an invader so that other cells know to immediately destroy anymore that they come across in the future. So that’s why if the same virus strand enters your body months later, it’s typically identified immediately and destroyed before it has the chance to multiply and make you sick again.
Ernest de Leon:
So is the problem that viruses just aren’t discovered by our immune system quickly enough, so they have enough time to multiply and invade the body before an immune response can actually take over?
Jolie Hales:
I think so.
Ernest de Leon:
I wonder if there are really obvious viruses that our body recognizes and destroys so quickly that we don’t even know we were infected.
Jolie Hales:
Yeah. Like they aren’t good at blending in with all the other cells, so the security alarm goes off right when they appear in the body or something. My guess is that it happens maybe with really wussy viruses. But then again, maybe those wussy viruses wouldn’t survive enough to be passed to another person at all. So natural selection might just eliminate them. I don’t know. But then there are other viruses that I know are especially deadly because they kill off our immune system before it can win the fight.
Ernest de Leon:
And this was the case with HIV for a very long time, until very recently where we’ve gotten the advanced therapeutics to actually deal with it.
Jolie Hales:
Exactly, exactly. That’s what made HIV, or makes HIV, such a dangerous virus, because after it invades the body, it immediately attacks and kills the T-cells, which is that immune system fleet that typically seeks out and destroys the invaders.
Newscasters:
It’s mysterious, it’s deadly, and it’s baffling medical science: Acquired Immune Deficiency Syndrome. If the trends continue as they are, I think we can predict that the Acquired Immune Deficiency Syndrome is a highly fatal illness likely to remain with us for the next decade.
Jolie Hales:
Okay. So with all this in mind, let’s go back to Rommie and what she and her team were trying to learn about Coronavirus.
Rommie Amaro:
We were very interested to study the main infection machinery of the virus.
Jolie Hales:
They were particularly focused on the part of the virus that actually latches onto human cells, causing an infection. And we’ve all actually seen images representing that molecular machine, whether we realize it or not.
Rommie Amaro:
The iconic image is the one that they show where it looks like a gray golf ball with like little red spikes coming out of it.
Jolie Hales:
Yeah, I see that all the time.
Rommie Amaro:
Right? So that’s the virus, and the red spikes that you see in all those images, that’s the actual spike protein. They actually call it the spike protein.
Jolie Hales:
Well, that makes sense.
Rommie Amaro:
Isn’t that cool?
Jolie Hales:
Finally, somebody in science that can name something the way I would have named it. There’s spikes here. Let’s call it a spike protein.
Rommie Amaro:
That’s exactly right.
Ernest de Leon:
Spoken like a true scientist.
Jolie Hales:
Always.
Rommie Amaro:
And each particular virus, you know, on average might have about 30 to 40 spikes per little gray ball. And that’s all it has, you know. Inside, it’s got all this other bits that it uses to carry out the infection process, but otherwise it’s like, you know, the virus is like a little container that has these spiky bits coming off of it. And it’s the spiky bits that ultimately encounter the host cell and come up and sort of touch or hit the host cell. And then that cell essentially can become infected.
Ernest de Leon:
So Rommie and her team were focused in on those spike proteins.
Jolie Hales:
Yes. And as a part of that, they wanted to learn more about how or why this particular virus was so good at infecting people — why it was able to latch on to human cells so effectively. And Rommie said that each COVID-19 virus basically has about a dozen different, what she calls “machines” on it that serve different purposes.
Rommie Amaro:
I’m studying just one of the machines, but I think it’s among the most important of the machines because it’s the machine that literally will latch onto your human cell and ultimately, like, infect you.
Jolie Hales:
What a jerk machine!
Rommie Amaro:
I know. It’s pretty dangerous. It’s very clever. I’m going to tell you what a clever machine this darn thing is, unfortunately for us and for our immune system.
Jolie Hales:
So through simulation, Rommie and her teams zoomed in on this particular machine, or this particular part of the virus spike protein.
Rommie Amaro:
If we could see what it looks like, and if we could understand how it moves, then maybe we could figure out a way to break the machine. And by breaking the machine, then we possibly could stop the virus from infecting human cells.
Jolie Hales:
So that was the goal– Figure out how this microscopic bully golf ball with a bunch of spikes on it is latching onto ourselves and making us sick and then use that information to destroy it.
Ernest de Leon:
Sounds like the work of an undercover superhero.
Jolie Hales:
And then, get this. She started to explain that apparently in our bodies, our cells are normally covered with this sort of sugary coating. And that admittedly got me really interested because anyone who knows me knows that I’m slightly and unfortunately obsessed with sugar. For instance, I love marshmallow peeps because they’re basically gelatinized sugar covered in crystallized sugar, which is amazing.
Ernest de Leon:
Probably not the same kind of sugar as the kind coating human cells.
Jolie Hales:
Sadly not. But I might just picture our human cells as a bunch of marshmallow peeps, anyway.
Ernest de Leon:
You do you.
Jolie Hales:
Oh, we could even combine… Oh, now I feel like I’m like in a children’s classroom or something, but what if we combine this space fighter visual with the marshmallow peep idea? Like, the immune system is an army of yellow marshmallow peep chicks in space fighters, taking out viruses.
Ernest de Leon:
Um, I think you’ve ventured into the territory of a child’s imagination.
Jolie Hales:
Okay, okay. But seriously, going back to the science of it all, the crazy thing that Rommie and her team have discovered relating to this sugary coating on our cells, is that the virus almost seems to know about it and use it against us. And how does it accomplish that? I’m going to tell you… After the break.
Jolie Hales:
They call it high performance computing, but is it really living up to its name? I mean, how much has really changed in the last 15 years? Since the revolutionary jump to cluster computing, there have been new core types, new ways to queue jobs, but no real seismic shift… Until now. Introducing Rescale, the intelligent control plane that allows you to run any app on any infrastructure, totally optimized. Innovators are moving away from the traditional data center-only model and stepping into the future where computing truly is high performance. Visit rescale.com/bcpodcast to learn what a modern approach to HPC can do for you. Rescale: Tomorrow’s HPC, today.
Jolie Hales:
Okay. Where were we?
Jolie Hales:
You just told us about the sugary shield on human cells.
Jolie Hales:
Yes. The marshmallow peep space fighters.
Ernest de Leon:
And you were just about to tell us how COVID fits into this picture.
Jolie Hales:
Yes. So, through their simulations, Rommie said that they likely discovered why COVID-19 is so good at infecting the human body. It turns out that the COVID-19 virus is also actually covered in its own sugary coating.
Rommie Amaro:
What the virus will do is it basically tries to hide itself from your immune system. And the way that it does this is by cloaking itself in a shield of sugar. And so by sort of covering all of its bad viral bits, I’ll call them, then the human immune system doesn’t sense that the virus is in your system. Instead, it just sees this sort of sugary coating and says, “Ohp, nothing to worry about. I’m gonna, you know, look for other invaders, you know, in your body.
Jolie Hales:
Crazy. It’s like camouflaging itself and tricking the immune system. Man, what a little sneaky sneak!
Rommie Amaro:
I know.
Ernest de Leon:
That is clever. COVID-19 is actually disguising itself as a marshmallow peep, so the fleet of immune system peeps doesn’t suspect the invader.
Jolie Hales:
Yes.
Ernest de Leon:
So, one thing we know about DNA and nature in general is that it is ultra efficient and it goes with what works. It’s very rare to find something that is truly unique and not applicable in any other way. So is this sugary coating unique to COVID?
Jolie Hales:
I asked Rommie the same question.
Rommie Amaro:
Many or most viruses do have this sugary coating, but they have it to different extents. This particular spike protein– This is pretty well-coated. It’s similar, I think, more to HIV than it is to the flu in that sense, in terms of like how much of the surface is sort of hidden by the sugary shield.
Ernest de Leon:
So that’s a very interesting comparison she draws here between the COVID-19 spike protein and the way HIV operates.
Jolie Hales:
Yeah. It sounds like there’s a lot of different viral particles that do the same thing, but COVID is, for some reason, especially good at it.
Rommie Amaro:
I mean, one of the reasons why we became interested in it as opposed to the structural biologist down the street or another scientist, is that we do, as I mentioned at the beginning, we do computational chemistry and computational biophysics. And one of the really interesting things about this sugary coating is that they can’t really take pictures of it. So experimentalists can’t take pictures of it and they can’t really figure out exactly what it looks like. So the only way to do that is using methods like ours– the sort of computational methods to basically predict sort of what the shield looks like in detail. So that’s one of the reasons we became interested because we realized, hey, there’s these people taking these amazing pictures of this little molecular machine, and they are fantastic pictures, but they don’t tell us the whole story. And if you don’t know the whole story, well, you know, part of the story is good, but we want to know as much as we possibly can about this darn virus, right? Because the more we know, the better we can equip ourselves to potentially fight it.
Jolie Hales:
And they’re fighting it by taking that detailed information about the holes in the sugary shield that reveal that there isn’t actually marshmallow or whatever underneath, or chinks in the armor, if you will. Then Rommie and her team provide that information to that large global scientific community fighting COVID. So even as we speak, the scientific community, including developers of vaccines and therapeutics, is using that very information from this very study to find ways to break through COVID sugary shield.
Ernest de Leon:
That’s an awesome impact right there.
Jolie Hales:
Right? I mean, imagine on the day when a vaccine is finally generally available, Rommie could quite possibly be at the health clinic waiting in line for a vaccine that her research actually helped create. And nobody around her will be the wiser that this woman standing right next to them has had such an impact on the world.
Ernest de Leon:
That’s what we call that undercover superhero.
Jolie Hales:
Is it not the perfect description for her? I mean, talk about a selfless field of work to go into.
Ernest de Leon:
And all of this is made possible through supercomputing.
Jolie Hales:
In fact, that’s the only way this sugary shield can really be observed– by creating a detailed visual through simulation. And so, Ernest, I wanted to show you a couple of these images that were computationally produced by Rommie’s team. And we’ll post these also in the show notes, as well, on bigcompute.org for our listeners who want to take a look at them themselves. Um, so here’s the first one. And I want you to describe since they can’t see it, describe to our listeners what it looks like to you.
Ernest de Leon:
First of all, the entire thing looks like something out of Yoshi’s Woolly World, but there’s like a base layer across the bottom that has a bunch of different colors on it. And then what appears, I’m guessing this is a spike protein coming out of it in a kind of a turquoise or teal color. And it looks like a, almost like a tree trunk. And then what would be branches filled with leaves of this, you know, cottony looking, actually cotton candy-looking teal thing.
Jolie Hales:
It’s interesting to see that spike protein that we’ve seen on the golf ball, zoomed in. This one’s blue instead of red. Um, it’s actually really beautiful the way that they’ve simulated it. And then there’s the second image, and tell us what it looks like in relation to the first image.
Ernest de Leon:
So the second image is literally the first, but the difference is it’s covered with these blue kind of fuzzball looking things, which I’m assuming are the sugar molecules that are coating the spike protein.
Jolie Hales:
Yes, you’re exactly right. So it’s basically the same image as before, except this one has that sugary coating. Exactly. And it does look like, like fuzzballs or like the craft store things that you glue on when you’re seven years old and you glue it on like your paper plates and give it to your dad for Father’s Day or something. It totally does look like that.
Ernest de Leon:
It does — almost like dark blue cotton balls stuck all over it.
Jolie Hales:
So, it’s pretty interesting to look at these images side by side, because first we have a closeup view of a COVID-19 spike protein, though his one, like we said, is blue instead of red. And then we have the same view, but with that sugary coating added to cover the surface of that spike protein.
Ernest de Leon:
And it’s really good to see the visualization here because now you can see what Rommie meant when she said that it really covers up the spike protein really well. There are obviously some areas that are still exposed, but by and large, the majority of the surface is covered. And when you say over the surface, it’s really over the surface. Barely any of the protein can be seen through that sugary shield.
Jolie Hales:
So we can see how it’s disguising itself to look like any regular sugar covered human cell marshmallow peep. And I mean, if I were part of the immune system patrolling the human body for intruders, I probably wouldn’t attack it.
Ernest de Leon:
That’s part of why COVID-19 is so contagious. It’s just too cleverly disguised to be detected faster.
Jolie Hales:
It looks like it. When the human body sees a COVID-19 virus, it basically just thinks it’s supposed to be there and then it doesn’t try to destroy it. So, then that virus latches onto the human cells with those little machines on the spike proteins, and then it starts to multiply, sometimes making the human host quite sick, but by using supercomputing–
Rommie Amaro:
We can actually in silico sort of make this model. And then what we do is we use the computer again and in a really sort of compute intensive way, we run these things called all atom molecular dynamics simulations, but basically, we can not only see what the sugar looks like, but we can also understand how it moves.
Jolie Hales:
They even published some short animations that show these simulated movements. Though, I honestly need a scientific interpreter to tell me what they mean. To me, it just looks like a lot of colorful springs and blobs jiggling around, but for a trained eye, these visuals are extremely valuable here. I’ll show you, Ernest. Maybe you’ll understand them more than I do.
Ernest de Leon:
Yeah. It’s hard for me to tell since I’m not a virologist or immunologist, but I can definitely see a lot of movement in this simulation showing where things are kind of attaching and how it’s attached in general. So it’s a really interestingly pool simulation to look at. And I’m guessing that it’s even more interesting if you’re a scientist in this field.
Jolie Hales:
That’s what I’m thinking.
Rommie Amaro:
One of the reasons why people before us weren’t able to see the full spike protein — what it looked like — is because these sugars, you know, when you think about the sugar on the marshmallow peeps that you love to eat, you think about like hard crystals, right?
Jolie Hales:
Yeah.
Rommie Amaro:
But this sugar moves. It moves more like the branches and leaves on a tree. And so it creates this, like, blurring effect and that’s part of its cloaking ability, or its camouflage. These sugars are kind of like sweeping around the surface of this molecular machine and thus making this sort of almost — more like a cloud of sugar, to protect it from the human immune system.
Jolie Hales:
What? That’s so crazy. If I saw marshmallow peep sugar doing that, it would freak me out.
Rommie Amaro:
You would eat it.
Jolie Hales:
Okay. Let’s be honest with ourselves.
Ernest de Leon:
Did she tell you how she ran the simulations?
Jolie Hales:
Yes.
Rommie Amaro:
We run these things called molecular dynamics simulations, which are these highly detailed physics-based dynamical models of systems. In this case, it’s a biological system. So what we do is we take that spike protein with all the sugars, and we embedded it in a viral membrane, and it’s this miniature three-dimensional model. And we approximate that model at the atomic level.
Jolie Hales:
So, I, of course, understood every word of that. But for those out there who might need some clarification, Ernest, do you care interpret?
Ernest de Leon:
Essentially, what she’s doing is modeling this at a very, very small, as she said, atomic level. And then she needs to take that and put it into motion.
Jolie Hales:
Ohhh. And I shouldn’t say that supercomputing is the only thing involved in research like this. So often it starts with hands-on experimentation and then computation actually amplifies the efforts and, and takes it further.
Rommie Amaro:
We start with a particular configuration that is specified by experimental data as much as possible. This is our like starting condition at T equals zero. And, you know, it’s, it’s all built. It’s got all the pieces, and then we do one integration step and we get another structure. We see how it’s moved in time. And this time step is really small. It’s like one or two femtoseconds only.
Jolie Hales:
What seconds?
Rommie Amaro:
Yeah. Do your listeners know about femtoseconds? They probably do.
Ernest de Leon:
Femtoseconds…
Jolie Hales:
Yeah, that was a new one for me. Apparently it’s 10 to the minus 15th of a second, or one-quadrillionth of a second. That’s a one with 15 zeros behind it.
Rommie Amaro:
So it’s a really, really, really small little stretch of time.
Jolie Hales:
Buckle up. It’s going to get technical.
Rommie Amaro:
But it has to be a really small stretch of time. Because if you take, if your integration step is too big, then you violate the physics of the system. You basically clash atoms into each other. And when you do that, then you’re totally screwed and your system basically blows up.
Jolie Hales:
Oh, geez, don’t want that.
Rommie Amaro:
Which is not good. No, you don’t want that. So basically, we perform this numerical integration on these big supercomputers, and we do this integration, millions and billions, and even trillions of times. And that’s how we build up taking very, very small steps, we can build up a dynamical trajectory of the system in time.
Jolie Hales:
Sorry, my brain just exploded.
Ernest de Leon:
So, for our listeners who may not be directly involved in this type of science, think of this like a motion picture. You know, before we had movies as we know them today, motion picture was exactly what it sounded like. It was a series of pictures that were taken in sequence often very rapidly, and then strung together to make it look like things were moving, even though it’s individual frames. This is exactly the same thing, just on a much, much smaller level and a much more contained or constrained set of data. So what machines were they using to run these simulations?
Jolie Hales:
Well, they were using a pretty well-known supercomputer–
Rommie Amaro:
A machine called Frontera, which is at the Texas Advanced Compute Center.
Jolie Hales:
And remember how the scientific community has come together in an unprecedented way to fight COVID-19? Well, the same thing can be said for the high performance computing industry.
Rommie Amaro:
Back in February, when we were just really seeing all the cases take off in Italy before it had hit the US, there might’ve been like less than 10 cases here — like early February. At that point, you know, we saw that it was a pretty big protein and it was going to take a lot of compute. So, I actually sent a quick email to Dan Stanzione, who’s the director of TACC, and I said, “Hey, Dan. Uh, you know, I hope you’re doing well. Uh, yeah, I don’t know what you guys are up to, but you know this virus — this looks, this virus is looking pretty serious. I think we probably need to do something with it.” And, again, this is really early days and he just said, “You know, Rommie, I totally hear you, and we’re going to make this machine available to you to use.”
Jolie Hales:
Wow.
Rommie Amaro:
Yeah, it was amazing. I mean, it was amazing to have that level of support. You know, in part, we’ve worked together on other projects before, but that was really key in sort of getting time to solution very quickly for this effort anyway. And I was so grateful for them.
Jolie Hales:
So the TACC provided the necessary compute resources for Rommie’s study, and they did it quite quickly.
Ernest de Leon:
In this community, this is what happens.
Jolie Hales:
Right.
Ernest de Leon:
So, I know that many tech companies also stepped up to join in this fight.
Jolie Hales:
You’re absolutely right. In fact, I was personally involved in the communications effort behind a program called Tech Against COVID. And it was basically where companies like Microsoft and Google, AWS and Rescale, which is a sponsor of this podcast — They had joined forces to provide free compute to scientists and researchers who were fighting COVID-19. And in order to get approval from them, it usually takes weeks, but we had four of these kinds of companies working together and they literally pushed out the program and the communication about it, in just a matter of hours. And I have, I’ve honestly never seen that before. And Tech Against COVID, wasn’t the only program out there that did this. I don’t know if you’ve heard of the COVID-19 HPC Consortium?
Ernest de Leon:
I have heard about them, but I have a feeling you’re going to tell us a little bit more about it.
Jolie Hales:
I am! I was on their website yesterday for this very reason. So the COVID-19 HPC Consortium is another resource hub that, and this one was actually spearheaded by the White House, where high performance computing resources are donated from again, a ton of different tech companies. So we’re talking Google and Nvidia, Microsoft, IBM, AWS, Hewlett Packard… There’s a whole bunch of them combined with academic supercomputing centers like TACC, as well as various government agencies and the Department of Energy National Laboratories. I mean, it’s a really big group, all donating resources.
Rommie Amaro:
Ordinarily, it takes us a really long time, like on the order of months, to get access to the big machines that you need to run these kinds of computations or these simulations. And one of the amazing things about COVID-19, or one of the, I guess, silver linings, is just that everybody decided to be much more cooperative and to step forward to support researchers to say, “Hey, if you have a need for compute, you can come to us. And we’re going to turn this around in like a couple of days or a few days, and give you as much as we can.”
Jolie Hales:
So as of this recording, the Consortium website says that there are 87 active projects. And I know that the Tech Against COVID program has active projects, as well. So it’s pretty neat. I know that with Rommie, the research that they’re doing is already being used across the globe.
Ernest de Leon:
And, in the HPC world, there’s a lot of buzz words we like to use — performance being one of them. But another one we love to talk about is scale. So with Rommie’s research, what kind of compute are we talking about?
Jolie Hales:
Okay. So for the spike protein study that we’ve mentioned here, they use 256 nodes per run with 56 cores per node, which if you do the math, ends up being 14,336 cores per run. And if you take a look at Frontera, which is considered to be one of the top 10 supercomputers in the world, it has 8,008 available compute nodes. So just to give you an idea of scope, it would have taken up around 3% of the entire Frontera machine at one time, just to do a run, to get this look at the spike protein sugars.
Rommie Amaro:
We were able to utilize a rather large chunk of it rather quickly, because some of our runs, we can actually sort of set up the different systems basically in parallel to each other. So we’re using multiple 256 node chunks at a time.
Jolie Hales:
And just to bring that home for someone who might be less familiar with supercomputing specs–
Rommie Amaro:
If we were trying to run this on a single desktop, which we can’t really — it’s not the best analogy because these particular runs, the way that the compute is done, you need to have these sort of big grabs of compute space because the problem is parallelized in a certain way, but it would be over… it would be probably over 700 years of equivalent of a single desktop. And I would say that’s probably what we use up in about two months.
Ernest de Leon:
So from 700 years down to two months. I’d say that’s a bit of a difference. And what software do they use?
Rommie Amaro:
So we use this amazing code called NAMD, which is a molecular dynamics engine nanoscale molecular dynamics engine, or N-A-M-D. It’s developed out of the University of Illinois at Urbana Champagne, where I got my PhD.
Jolie Hales:
Oh, nice.
Rommie Amaro:
Yeah, yeah. That’s cool — connection.
Jolie Hales:
And not only were they using NAMD software, but they got to help kind of customize it as they went, which is unique.
Rommie Amaro:
We worked together, not only with the team at TACC on the hardware, but then of course also we worked really closely with the software developers, like the NAMD team. There’s Jim Phillips and John Stone, and David Hardy — a whole bunch of folks who are working to tune and optimize the code to these particular architectures. And that also makes a big difference, you know, just trying to squeak a couple more flaps per run gives us a great advantage. So we’ve worked really well, I think, as a team altogether in those aspects.
Ernest de Leon:
If there’s one thing you know about the software development world, it is that as time has gone on and compute power has increased, there’s been a general movement away from software efficiency. So it’s amazing to watch or hear that Rommie was able to work directly with the writers of the software to actually improve its performance on the specific supercomputer she was using so that she could squeak out just a tiny bit more performance to make the simulation run a little bit faster.
Rommie Amaro:
And to go a little deeper for you, Ernest, the code is in C++, and then it uses this thing called Charm++ for the parallelization. And then most of our work, we sort of interface through TCL, or through T-C-L interfaces.
Ernest de Leon:
Now that’s not too surprising to hear, because again, in the software development world, we use a lot of interpreted languages nowadays for a bunch of different things. But when we really need a heavy hitter in terms of performance, we go to compiled languages. And specifically, we want to get really low, so we go into C++.
Jolie Hales:
And as they’re doing all of this, they thought, why stop at just looking at the spike protein when there’s obviously so much more of this virus — this golf ball with spikes on it that can be explored through computational simulation.
Rommie Amaro:
The other thing that we’re working on, which we haven’t published yet, but we’re really working hard on is actually moving beyond studying the single spike to actually simulating the whole virus. And so, you know, that takes our problem up in order of magnitude or more. And so there, we do have some prototype systems up and running now on Frontera of the full virus, and there we’re using 2,048 nodes. So, you know, over 2,000 nodes of the machine.
Ernest de Leon:
Wait — 2,048 nodes of an 8,000 node machine. If they’re running that all in parallel, we’re talking about over a quarter of the machine.
Jolie Hales:
All for a virus that is one-900th the width of a piece of hair.
Rommie Amaro:
We are learning things that really you cannot see in other ways. There really is no alternate experiment that could be performed that would give you what we can provide with these simulations. Like it is really unique. And that’s, you know, one of the reasons why it’s so cool and has kind of made a splash is because, you know, we showed for the first time what it looks like. People hadn’t seen that before, and they couldn’t without compute. So simulations, in that sense, have a very unique role to play in this sort of space of research in many different areas, but especially in the case of COVID-19.
Jolie Hales:
And while computing is doing a lot, there is still opportunity on the horizon to do even more.
Rommie Amaro:
There are still sort of gaps in our capabilities in terms being able to simulate, for example, long timescales or for very complex scenes that have like many molecular piece parts. I think combining these types of simulations together with artificial intelligence, new methods coming in artificial intelligence, that’s something that really is just starting to sparkle on the horizon.
Ernest de Leon:
That’s amazing. And artificial intelligence is one of the areas that I really love to be involved in, think about, especially in the HPC context. And it’s, for reasons like this, artificial intelligence is what’s going to allow us to shift from kind of what we know and what we do, and looking in on something that has already existed or currently exists to predictive analytics — being able to predict something that will happen or predict of movement of something.
Rommie Amaro:
I’m one of, probably many people, who really believe that the real breakthroughs in research are going to come at the intersection of simulation sciences with experimental observational science. That’s sort of what I was getting at in the last sort of example, with AI. But when we can combine what we can measure with accurate models that can predict forward, then we can get ahead of a lot of these problems, you know? And we could also maybe have a chance to do things like try to thwart climate change or to develop better sustainable energy methods and materials, or develop new therapeutics. I think that’s the direction that I’m most excited about.
Jolie Hales:
So with everything Rommie and her team have learned so far, I asked her what advice she would give her friends and her family, or what she tells her friends and family when it comes to COVID-19.
Rommie Amaro:
Wear a mask.
Jolie Hales:
Oh yeah, for real.
Rommie Amaro:
Cause really that’s one of the things, I mean, it ties into that because it is a very clever virus and it has a lot of ways of being clever, a lot of ways that we don’t understand, but this just goes to show you don’t want to catch this. And it’s like, to me, when I first saw this, it was like, “Oh my God, this is a very smart virus. And it’s even yet another reason to wear a mask, and just to be so careful and just to continue to be vigilant. We feel like we’ve been at this a long time, right? March 13th was a long time ago. It’s like 200-some days. Everybody’s getting burned out. But the thing is, the virus doesn’t get fatigued. We still do not have immunity. It is still super smart with a great camouflage mechanism. So I don’t know. I mean, it’s tough, but we’ve got to stay the course.
Jolie Hales:
Stay the course, Ernest, or risk getting sick via marshmallow peep imitation.
Ernest de Leon:
[laughs]
Jolie Hales:
I feel like I’m going to dramatically regret the marshmallow peep part of this whole episode. Later, when I listen to it, I’m gonna be like, “Oh, why, why did I talk about marshmallow peeps every five seconds?”
Ernest de Leon:
I wouldn’t be surprised if there wasn’t a peep about it in the final cut. [ba dum, ching]
Jolie Hales:
So, Ernest, let’s review what we’ve learned through this episode.
Ernest de Leon:
I’ve actually picked up on a lot in this interview, and I’m going to take it back to probably my favorite part, which is her explanation of the molecular dynamics and kind of putting that into motion at the femtosecond and kind of tying it with the whole motion picture thing, right? And that’s really, what’s critical here is that scientists are able to understand not only what this thing looks like in a frozen state or a snapshot in time, but also how does it move? How does it interact with other cells? And you really cannot see that without supercomputer.
Jolie Hales:
It’s true. I mean, while it’s not cool that the pandemic is in our midst right now, it’s better now than it would have been before this technology existed.
Ernest de Leon:
Imagine what it was like in 1918 when the last pandemic came.
Jolie Hales:
Yes. We’ve learned about how these sugars are disguising the virus to look like any of our other human cells. And it just goes to show that, I mean, every virus is different out there and I’m sure there’ll be another one that’s going to be clever and sneaky, like Coronavirus coming down the pipe. So I’m grateful for the scientific research that we have and the supercomputing that can take us to new heights. So if you want to learn even more about these studies, you can check out the episode notes on bigcompute.org, where we’ll drop some links and some pictures and such for you. You can also go to Amarolab.ucf.edu for more technical information, and you can follow Rommie on Twitter via @RommieAmaro, and that’s R-O-M-M-I-E — two Ms. Rommie Amaro.
Ernest de Leon:
And we invite you to follow Big Compute on social media, on LinkedIn, Twitter, Facebook, and YouTube.
Jolie Hales:
Yes. All of our social media accounts are somewhat new and we’re especially brand spanking new on YouTube, like literally a few days ago. So please go show us some love. I think we have nine subscribers and they’re probably all my mom.
Ernest de Leon:
And we wanted to give special thanks to Rommie Amaro and her awesome team at UC San Diego, as well as Dan and TACC, the NAMD software creators, the COVID-19 HPC Consortium, Tech Against COVID, and the teachers and people who have inspired these researchers to do what they’re doing.
Jolie Hales:
Yes. Oh, in fact, there was a professor Rommie mentioned by name who inspired her to study chemistry when she was an undergrad and his name was…
Rommie Amaro:
Steve Zumdal.
Jolie Hales:
So like these scientists, researchers, and engineers, were grateful for the teachers out there who are making a difference.
Ernest de Leon:
And to all of you out there who are conducting research or are on the front lines of this virus, a special thanks and shout out to each of you from us at Big Compute.
Jolie Hales:
That’s going to do it for this episode of the Big Compute Podcast. I think this is where we insert a plea to leave us a five star review, but I feel stupid asking for it.
Ernest de Leon:
I’ll do it. Leave us a five-star review on Apple Podcasts.
Jolie Hales:
Yay! Or wherever you listen to your podcasts.
Ernest de Leon:
See you next time.
Jolie Hales:
Bye!







